
Repairs within a cell
A new study by the research group led by Laura Aradilla Zapata, Professor for Molecular Cell Biophysics an der Universität des Saarlandes, shows the central role of a protein called “tau” in repairing the cell skeleton.
Publication: Biswas, S., Grover, R., Reuther, C. et al. Tau accelerates tubulin exchange in the microtubule lattice. Nat. Phys. (2025).
See also press release by Saarland University (4.9.2025)

Fast regulation of calcium level
In collaboration with Prof. Dr. Bernd Fakler from the University of Freiburg, Heiko Rieger, Professor für Statistische Physik an der Universität des Saarlandes, highlights the central role of calcium pumps in regulating intracellular calcium concentrations. These pumps operate at a transport speed more than 100 times higher than previously assumed.
Publication: Cristina E. Constantin, …, Heiko Rieger & Bernd Fakler: Ca2+-pumping by PMCA-neuroplastin complexes operates in the kiloHertz-range. Nature Communications16, 7550 (2025).
See also press release by Saarland University (26.8.2025)

From benign to aggressively growing brain tumor
A team led by Professors Joachim Oertel, Director of the Clinic for Neurosurgery at Saarland University Hospital, Steffi Urbschat, Head of the Research Laboratory at the Clinic for Neurosurgery, and Gregor Jung, Professor of Biophysical Chemistry at Saarland University, have now succeeded in developing a coloring method that costs little and only takes a few minutes.
Press release by Saarland University from 11.02.2025 (in German)

The structure and mechanics of the cell cortex depend on the location and adhesion state
The actomyosin cortex plays a crucial role in determining cell mechanics and thus a variety of cell functions such as migration, division and differentiation. Understanding the relationship between the structure and mechanics of the cortex in different situations is necessary to explain cell properties that are crucial for health and disease, e.g. cancer. A team led by biophysicist Franziska Lautenschläger has investigated a clear correlation between the structure and stiffness of the cortex, taking an important step towards predicting and controlling the mechanical behavior and thus the function of cells.
The work was carried out as part of the Collaborative Research Center 1027 “Physical Modeling of Nonequilibrium Processes in Biological Systems”
Publication:
D. A. D. Flormann, ..., F. Lautenschläger: The structure and mechanics of the cell cortex depend on the location and adhesion state, Proc. Natl. Acad. Sci. U.S.A. 121 e2320372121 (2024).
https://www.pnas.org/doi/10.1073/pnas.2320372121
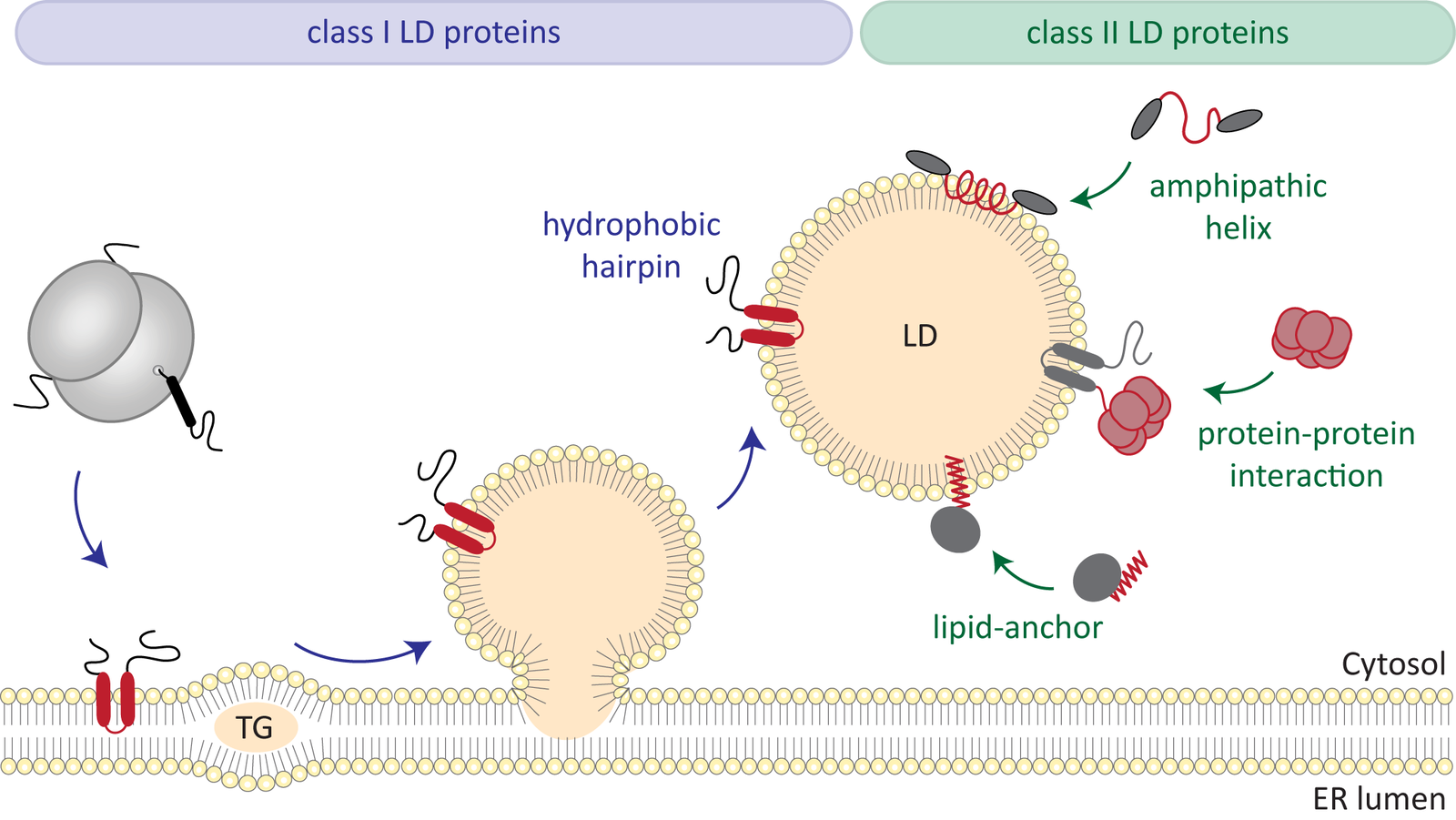
New insights into fat metabolism
Fats, the most important energy stores in the human body, are stored in so-called lipid droplets in the cells. Very little is known about how they work. A team led by biologist Bianca Schrul from Saarland University has now gained insights into a specific protein in the shell of these lipid droplets. This opens the door for further research, which could also help to better understand metabolic diseases.
The work was carried out as part of the Collaborative Research Center 1027 “Physical Modeling of Nonequilibrium Processes in Biological Systems”
Publication:
R. Dhiman, R.S. Perera, C.S. Poojari, H.T.A. Wiedemann, R. Kappl, C.W.M. Kay, J.S. Hub, B. Schrul: Hairpin protein partitioning from the ER to lipid droplets involves major structural rearrangements. Nat. Commun. 15, 4504 (2024).https://doi.org/10.1038/s41467-024-48843-8
Press release of Saarland University from 12.06.2024 (in German)

Vesicles driven by dynein and kinesin exhibit directional reversals without regulators
Intracellular vesicular transport along cytoskeletal filaments ensures targeted cargo delivery. Such transport is rarely unidirectional but rather bidirectional, with frequent directional reversals owing to the simultaneous presence of opposite-polarity motors. So far, it has been unclear whether such complex motility pattern results from the sole mechanical interplay between opposite-polarity motors or requires regulators.
In our recent publication in Nature Communications, we - together with the group of Stefan Diez (TU Dresden) - demonstrate that a minimal system, comprising purified Dynein-Dynactin-BICD2 (DDB) and kinesin-3 (KIF16B) attached to large unilamellar vesicles, faithfully reproduces in vivo cargo motility, including runs, pauses, and reversals. Remarkably, opposing motors do not affect vesicle velocity during runs. Our computational model reveals that the engagement of a small number of motors is pivotal for transitioning between runs and pauses. Taken together, our results suggest that motors bound to vesicular cargo transiently engage in a tug-of-war during pauses. Subsequently, stochastic motor attachment and detachment events can lead to directional reversals without the need for regulators.

Adhesiveness of Staphylococcus aureus cells is unevenly distributed across the cell surface
Staphylococcus aureus, a dreaded hospital germ, can cause serious infections. One remarkable characteristic of this bacterium is its extraordinary adhesive ability. A team from the Physics and Medicine departments at Saarland University has now discovered that the germ adheres better to some parts of the envelope than to others. Their study, which has now been published, could be the starting point for more effective bacteria-repellent surfaces.
Press release of Saarland University (17.10.2023)
Publication:
C. Spengler, E. Maikranz, B. Glatz, M.A. Klatt, H. Heintz, M. Bischoff, L. Santen, A. Fery and K. Jacobs: “The adhesion capability of Staphylococcus aureus cells is heterogeneously distributed over the cell envelope”, Soft Matter, 2023, Advance Article.



