Research
The biomedical research in the Rother laboratory focuses on signaling pathways regulated by glycosaminoglycans. Diseases and pathologic conditions associated with altered glycosaminoglycan assembly like inflammation, chronic wounds and cancer are serious world-wide health problems significantly reducing the patients’ quality of life. Glycosaminoglycans cover and surround all cells as part of the cell membrane and within the extracellular matrix. These long, unbranched polysaccharides are key molecules in tissue development, homeostasis and wound healing. Due to their ability to bind a variety of proteins like growth factors, cytokines and extracellular matrix proteins, these sugars direct and orchestrate specific functions as well as the localization of these bioactive proteins.

Currently, we investigate the regulation of the glycosaminoglycan composition and functionality by growth factors of the TGF-β cytokine family in the context of dysregulated molecular signaling in tumor cells, which result in altered cell behavior. In addition, artificial extracellular matrices in form of biomimetic implant coatings and hydrogels functionalized with glycosaminoglycans are developed and used as biomimetic 2D and 3D cell culture systems to analyze how mediator proteins and the cell microenvironment influence the cellular response.
Our group aims to answer the following questions:
- How are processes like the dynamic extracellular matrix remodeling and growth factor signaling coordinately regulated by the specific structure and substitution patterns of glycosaminoglycans?
- Which structural modifications of the polysaccharide chains of glycosaminoglycans are important to regulate the receptor recognition and signal transmission of bioactive proteins?
- What are the consequences of altered glycosaminoglycan-protein binding profiles on cell activation as well as on direct cell-cell and cell-matrix interactions depending on tissue and pathogenesis stages?
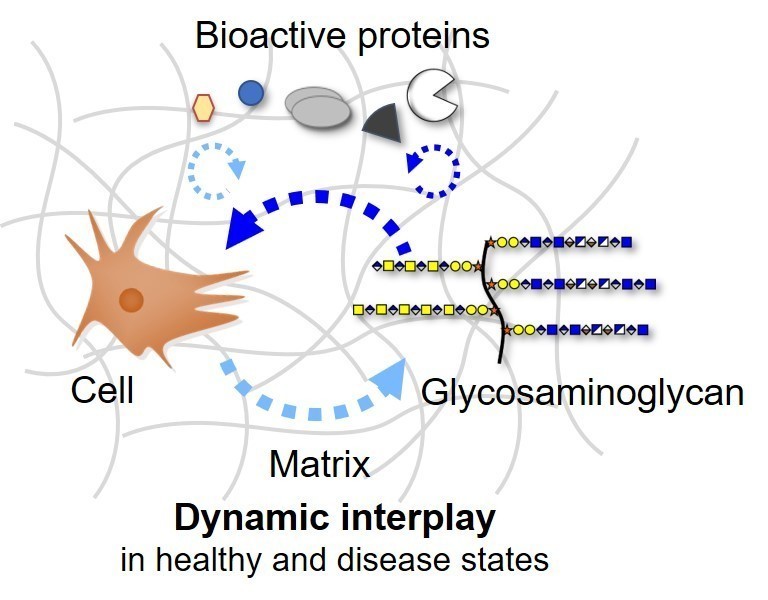
With a deeper molecular understanding of dysregulated signaling processes involving glycosaminoglycans, new diagnostic markers can be identified with the potential for future innovative intervention strategies. The work in the Rother lab includes amongst others sensor-based interaction studies in real-time (Surface Plasmon Resonance, SPR), Fluorescence Activated Cell Sorting (FACS), glycosaminoglycan composition analysis via LC-MS and matrix engineering. In vitro studies on mutant cell lines generated using CRISPR/Cas9 gene targeting combined with artificial extracellular matrices allow us to analyze the glycan composition and functionality in health and disease states.
