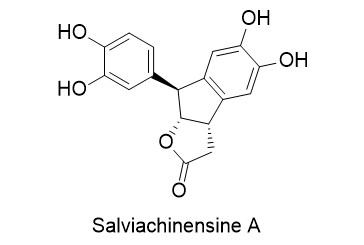
Salviachinensine A
In 2018, Wang et al. reported the isolation of six new phenolic acid derivatives, called salviachinensines, from the Chinese medicinal plant Salvia chinensis. Salviachinensine A was found to be the most active compound, displaying antiproliferative properties and inducing apoptosis and the arrest of cell cycle progression in acute myeloid leukaemia cell lines MOLM-13 and MOLM-14 (IC50: 2.3 and 7.1 µM, respectively).
- Q. Wang, Z. Hu, X. Li, A. Wang, H. Wu, J. Liu, S. Cao, Q. Liu, J. Nat. Prod. 2018, 81, 2531−2538.
Salviachinensine A was synthesised for the first time by employing Donald Matteson’s boronic ester homologation. The use of (R,R)-dicyclohexylethane-1,2-diol (DICHED) enabled the generation of the required stereogenic centres in a highly stereoselective fashion. Salviachinensine A was formed in a one-pot protecting group cleavage/lactonisation/ intramolecular Friedel-Crafts alkylation, followed by Lewis acid-catalysed cleavage of the acetal protecting groups.
- M. Kempf, O. Andler, U. Kazmaier, "Total synthesis of Salviachinensine A using Matteson's homologation approach", Helv. Chim. Acta 2023, e202300136. DOI: 10.1002/hlca.202300136.